The FDA Office of Generic Drugs (OGD) is the engine behind every generic medication you pick up at the pharmacy. It’s not a behind-the-scenes footnote-it’s the reason you can buy a life-saving drug for $5 instead of $500. While most people think of the FDA as just approving brand-name drugs, the truth is that nearly 9 in 10 prescriptions filled in the U.S. are for generics. And none of that happens without OGD.
The Office of Generic Drugs is a division within the FDA’s Center for Drug Evaluation and Research (CDER). It wasn’t always this way. Before December 2013, generic drug reviews were scattered across different teams, leading to delays and confusion. That changed when the FDA restructured OGD into a "super office"-a standalone unit reporting directly to the CDER director. This wasn’t just a name change. It was a full upgrade in authority, resources, and focus.
OGD’s mission is simple: ensure that safe, effective, and high-quality generic drugs reach patients as quickly as possible. It doesn’t just review applications-it builds the rules, sets the standards, and coordinates global efforts to keep the system running. Its work touches every person who takes a generic drug, whether it’s blood pressure medicine, antibiotics, or insulin.
OGD isn’t one big team. It’s made up of six key parts: the Immediate Office and five specialized sub-offices. Each has a clear job, and together they form a machine designed to move thousands of drug applications every year.
OGD doesn’t just sit and wait for applications. It drives the entire generic drug pipeline. Here’s what it really does:
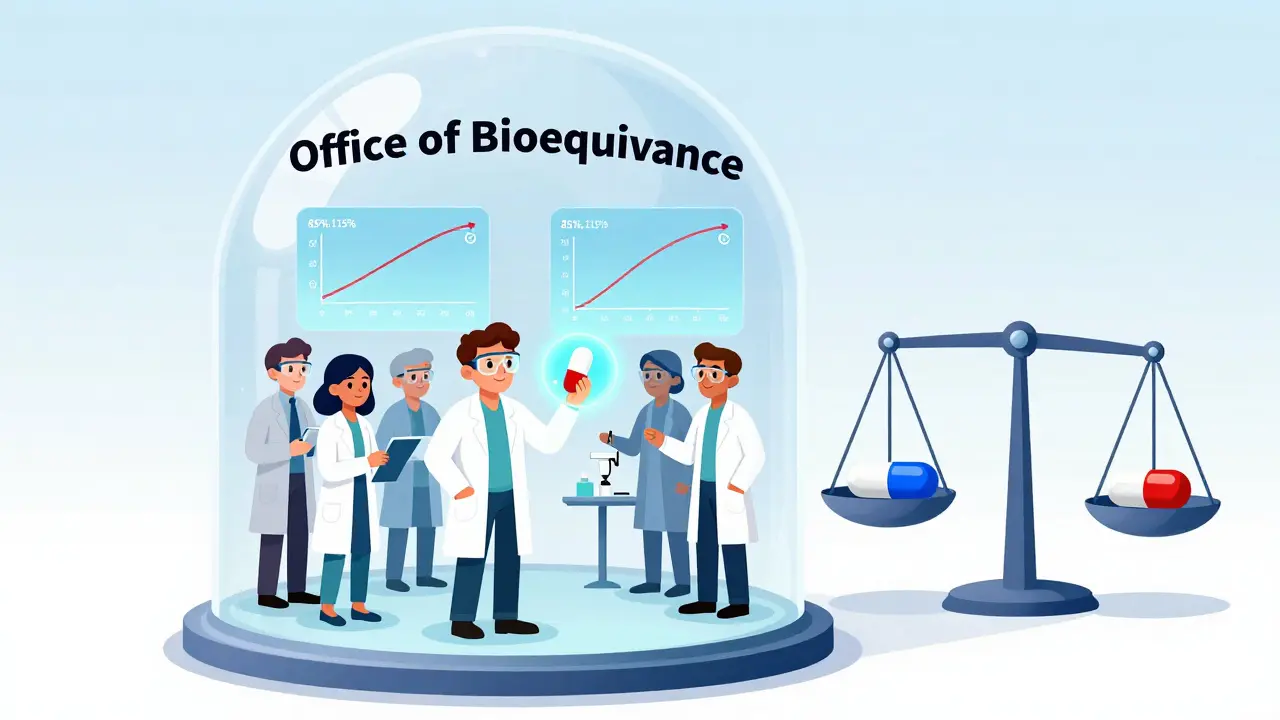
Many people think generics are "inferior" because they cost less. That’s not true. The science is clear: a generic drug must be bioequivalent to the brand. That means:
OGD uses advanced testing to prove this. For example, a generic version of a blood pressure pill must release its drug into the bloodstream within 85% to 115% of the brand’s absorption rate. That’s not a guess-it’s a scientifically validated range. If a generic falls outside it, OGD rejects it.
Some drugs are harder to copy. Complex formulations like inhalers, patches, or long-acting injectables require more advanced testing. That’s where OGD’s Office of Research and Standards comes in-they develop new methods to test these tricky products so they can be approved without compromising safety.
Every year, OGD approves over 1,000 generic drugs. In 2023 alone, generic medications saved the U.S. healthcare system more than $370 billion. That’s not a number-it’s real money in people’s pockets. A patient on insulin might pay $20 a month instead of $300. A cancer patient might get a life-extending drug because their insurance won’t cover the brand version.
OGD also protects you. When a generic drug is approved, it’s not just a cheaper copy. It’s held to the same quality standards as the brand. OGD inspects manufacturing facilities-both in the U.S. and overseas-to make sure they meet FDA standards. If a facility fails inspection, OGD blocks approval until it’s fixed.
And when something goes wrong? OGD is the first to respond. If reports come in that a generic is causing unusual side effects, they investigate. They compare data to the brand. They check for contamination. They talk to manufacturers. They don’t wait for a crisis-they act before it spreads.
OGD doesn’t work alone. It coordinates with other FDA offices like the Center for Biologics Evaluation and Research (CBER) for biologics, and the Center for Veterinary Medicine for animal drugs. It also works with the National Institutes of Health (NIH) on research and the Centers for Medicare & Medicaid Services (CMS) on pricing impacts.
It’s also a bridge between industry and public health. When a company submits an ANDA, OGD doesn’t just say yes or no. It gives feedback, clarifies requirements, and helps manufacturers understand what’s needed. That’s why the approval rate for generics has steadily improved since 2013-because the process is clearer, faster, and more predictable.

Approval is just the beginning. Once a generic hits the market, OGD continues to monitor it. They track adverse events through the FDA’s MedWatch system. They analyze data from pharmacy chains and hospitals. They watch for manufacturing changes-like switching suppliers or changing the tablet coating-that could affect how the drug works.
If a problem is found, OGD can require label changes, issue safety alerts, or even request a recall. This ongoing oversight is what makes the system trustworthy. You don’t have to wonder if your generic is safe. OGD ensures it is.
| Sub-Office | Primary Function | Key Teams or Units |
|---|---|---|
| Immediate Office (IO) | Leadership, strategy, global coordination | Global Generic Drug Affairs Team, Division of Legal and Regulatory Support |
| Office of Bioequivalence (OB) | Proving drug absorption matches brand | Division of Clinical Review, OGD Safety and Surveillance Team |
| Office of Generic Drug Policy | Interpreting laws, setting rules | Division of Policy Development, Hatch-Waxman experts |
| Office of Regulatory Operations (ORO) | Managing ANDA reviews and deadlines | Regulatory Project Managers (RPMs), Division of Project Management |
| Office of Research and Standards (ORS) | Developing testing methods and standards | Division of Therapeutic Performance, Division of Quantitative Methods |
| Office of Safety and Clinical Evaluation | Monitoring post-market safety | Adverse event analysis, manufacturing inspection coordination |
As more complex drugs come off patent-like biologics and combination therapies-OGD is preparing for the next wave. It’s investing in new technologies, training staff on advanced analytics, and expanding global partnerships. The goal? To keep generic drugs accessible, affordable, and safe-even as the science gets harder.
For patients, that means more choices. For insurers, lower costs. For the system, more sustainability. OGD isn’t just a government office. It’s a public health lifeline.
Yes. By law, a generic drug must have the same active ingredient, strength, dosage form, and route of administration as the brand. It must also be bioequivalent-meaning it delivers the same amount of medicine into the bloodstream at the same rate. The FDA requires rigorous testing to prove this. If a generic doesn’t meet these standards, it’s not approved.
The active ingredient is identical, but inactive ingredients-like color, flavor, or filler-can differ. These don’t affect how the drug works. The FDA allows these differences because they don’t impact safety or effectiveness. The pill’s shape or color may change if a different manufacturer makes it, but the medicine inside is the same.
Under GDUFA, OGD aims to review 90% of first-time applications within 10 months. Complex applications or those with data issues may take longer. First generics and drugs in shortage are prioritized and often reviewed faster. On average, the entire process-from submission to approval-takes 12 to 18 months, but many are approved in under a year.
Not always. Over 80% of the active ingredients in U.S. generic drugs come from overseas, mostly India and China. But every facility-whether in the U.S., India, or elsewhere-must pass FDA inspections. OGD inspects these sites regularly and can refuse approval if standards aren’t met. Quality, not location, determines approval.
Yes. If post-market data shows a generic drug is contaminated, ineffective, or causes unexpected side effects, OGD can require a recall. This has happened multiple times-for example, when certain blood pressure generics were found to contain trace amounts of carcinogens. OGD acts quickly when safety risks are confirmed.
An NDA (New Drug Application) is for brand-name drugs and requires full clinical trials to prove safety and effectiveness. An ANDA (Abbreviated New Drug Application) is for generics and doesn’t require new clinical trials because it relies on the brand’s data. Instead, it must prove bioequivalence. The ANDA process is faster and cheaper, which is why generics cost less.
Yes. All generic drugs sold in the U.S. must be approved by OGD. Even if a drug is made overseas, it can’t be sold here without an approved ANDA. OGD is the only U.S. agency with the legal authority to approve generic drugs for sale.
You don’t need to know how OGD works to take your medicine. But you should know it’s there-working quietly, every day, to make sure what you’re taking is safe, effective, and affordable. Behind every low-cost pill is a team of scientists, regulators, and policy experts who didn’t just check a box. They built a system that saves billions and keeps millions of people healthy.