
Generic drugs are just as effective as brand-name drugs, with identical active ingredients and proven bioequivalence. The FDA requires them to match in strength, dosage, and safety - only the price and appearance differ. Learn what the label really means.
READ
Learn how to effectively communicate with your doctor about staying on brand medication instead of generics. Includes preparation tips, communication strategies, insurance considerations, and common scenarios where brand is necessary.
READ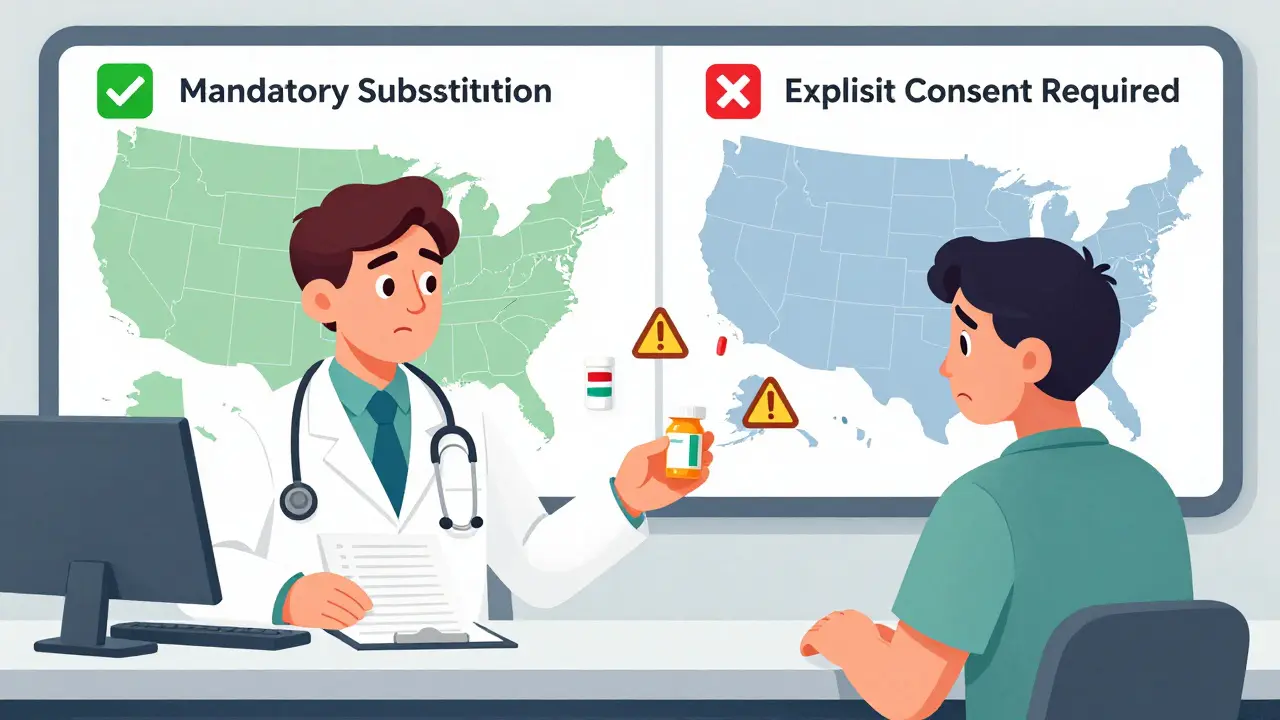
State laws on generic drug substitution vary widely across the U.S., affecting how pharmacists swap brand-name drugs for cheaper generics. Learn how consent rules, NTI drug restrictions, and biosimilar policies impact your prescriptions and what you can do to protect your health.
READ
Drug patents last 20 years from filing, but most drugs only have 7-12 years of market exclusivity due to long approval times. Extensions, regulatory barriers, and patent stacking can delay generics for years - here's how it really works.
READ