State Laws on Generic Drug Substitution: What Pharmacists and Patients Need to Know
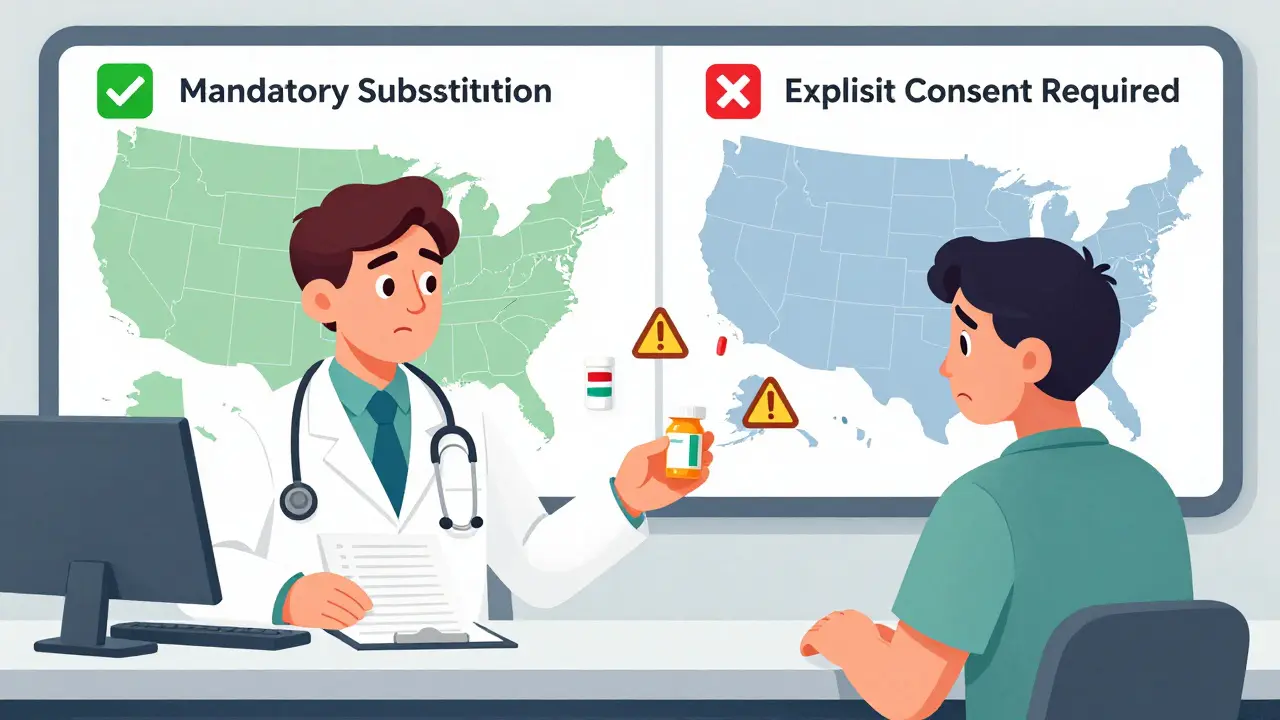
When you pick up a prescription, you might not think about whether the pill in your hand is the brand name or a cheaper generic version. But behind that simple swap is a tangled web of state laws that vary wildly across the U.S. These rules determine if a pharmacist can switch your brand-name drug for a generic - and whether they need your permission to do it. In some states, the pharmacist must substitute. In others, they can’t do it unless you say yes. And in a few places, certain drugs are off-limits for substitution altogether, even if they’re technically the same.
Why State Laws Even Exist
The push for generic substitution started in the 1980s after the Hatch-Waxman Act gave the FDA a clear way to approve cheaper versions of brand-name drugs. The goal? Save money without sacrificing safety. Generic drugs now make up 92.5% of all prescriptions filled in the U.S., saving the system over $300 billion a year. But savings alone didn’t drive the laws. States stepped in because they saw the potential for mistakes - especially with drugs where even tiny differences in how the body absorbs the medicine can cause harm.
These are called narrow therapeutic index (NTI) drugs. Think warfarin for blood thinning, levothyroxine for thyroid function, or certain seizure medications. A 5% difference in absorption might mean the difference between control and a dangerous seizure or a stroke. That’s why some states treat them differently.
Four Ways State Laws Differ
Not all states play by the same rules. There are four big areas where laws diverge:
- Duty to substitute: In 22 states, pharmacists are legally required to swap the brand for the generic unless the doctor says "dispense as written" or the patient refuses. In the other 28 states and D.C., substitution is optional - the pharmacist can choose whether to swap or not.
- Patient consent: Thirty-two states use "presumed consent." That means the substitution happens automatically unless you say no. Eighteen states require "explicit consent" - you have to actively agree before the switch.
- Notification: Forty-one states require pharmacists to tell you after they’ve made the switch. This could be a note on the label, a verbal reminder, or a printed sheet. In the other nine, they don’t have to say anything.
- Liability protection: Thirty-seven states shield pharmacists from lawsuits if they follow the rules correctly. If you get sick after a substitution in one of those states, the pharmacist likely won’t be held responsible - as long as they did everything the law asked.
What Happens with Biosimilars?
Biosimilars are the next generation of generics - they’re not exact copies like traditional generics, but close enough to work the same way. They’re used for complex drugs like those for cancer, arthritis, or autoimmune diseases. As of 2023, 49 states and D.C. have laws covering biosimilar substitution. But the rules are messy.
Hawaii is the strictest: you need both your doctor’s approval and your own written consent to switch a biosimilar for an antiepileptic drug. Florida requires pharmacies to maintain a formulary that proves the substitution won’t endanger patients. Iowa tells pharmacists to stick with the FDA’s Orange Book - the official list of approved equivalents. And 12 states updated their laws in 2023 to match the FDA’s new "interchangeable" designation, which gives biosimilars a higher level of confidence.

Real-World Problems
The patchwork system creates confusion - especially for people who live near state borders or travel often. A patient in New York might be asked every time if they want the generic. Their neighbor in New Jersey gets the generic automatically unless they object. When that patient visits the other state, they get different treatment. It’s frustrating, and it leads to mistrust.
Patients report feeling like guinea pigs. Between 2020 and 2022, the FDA received 217 reports of problems after generic switches - mostly involving levothyroxine and warfarin. One woman in Minnesota switched from brand-name warfarin to a generic and ended up with a dangerous blood clot. Her doctor had warned her about the risks, but the pharmacist followed state law and substituted anyway.
Pharmacists are stuck in the middle. A 2022 study found they spend an average of 12.7 minutes per prescription just checking state rules, FDA listings, and patient history. Chain pharmacies with locations in multiple states rely on software that auto-checks substitution rules - 83% now use these tools. But independent pharmacies? Many still flip through printed state law guides or call the board of pharmacy.
Who Benefits? Who Loses?
The savings are real. States with mandatory substitution see 12.3% higher generic fill rates for drugs like statins. Medicaid programs save an estimated $1.2 billion a year just from these laws. For patients on fixed incomes, that means lower co-pays and fewer skipped doses.
But the cost isn’t just financial. Patients with rare diseases, epilepsy, or heart conditions often fear substitution. A 2023 survey by the Life Raft Group found 41% of cancer patients worried about switching NTI drugs. Many doctors now write "dispense as written" on prescriptions - not because generics are unsafe, but because they’ve seen patients suffer after a switch.
And here’s the irony: the FDA says all Orange Book-listed generics are therapeutically equivalent. But doctors and patients still report differences. Dr. Robert Temple, former FDA official, admitted in 2019 that some patients do experience changes - even if the science says they shouldn’t. The body isn’t a lab. Individual biology matters.
What You Can Do
If you’re on a critical medication - especially warfarin, levothyroxine, seizure meds, or immunosuppressants - don’t assume the pharmacy will tell you about a switch. Ask:
- "Is this the same drug I got last time?"
- "Was this a generic substitution?"
- "Do I need to tell my doctor if it changed?"
If you’re uncomfortable with the switch, say no. You have the right. Even in mandatory substitution states, your refusal overrides the law.
Keep a list of your medications - brand name and generic - and update it every time you refill. Share it with every new doctor. If you notice new side effects after a switch, report them to your pharmacist and doctor. You can also file a report with the FDA’s MedWatch system.
The Future: Standardization or Chaos?
The American Pharmacists Association says 78% of pharmacists are confused by the current patchwork. The National Conference of State Legislatures has a scoring system - Louisiana scores 0 (most friendly to substitution), Hawaii scores 4 (least friendly). That’s not a system. It’s a maze.
The Uniform Law Commission is working on a model law to harmonize biosimilar rules across states. The Congressional Budget Office estimates that if all states aligned their substitution policies, the U.S. could save another $8.7 billion by 2028.
But patient advocates warn that forcing uniformity could hurt vulnerable groups. The National Organization for Rare Disorders argues that one-size-fits-all rules ignore the real risks for people with rare diseases.
Right now, the system works because it’s flexible. But it’s also broken because it’s inconsistent. Until states agree on a common standard - or Congress steps in - the burden falls on you: the patient. Know your rights. Ask questions. Don’t let convenience override your safety.
Can a pharmacist substitute my brand-name drug without telling me?
In 41 states, pharmacists must notify you after substituting a generic. But in the other nine states, they don’t have to say anything. Even in states that require notification, the notice might be a small label on the bottle or a printed sheet - easy to miss. Always check your medication and ask if it changed.
Are generic drugs really the same as brand-name drugs?
The FDA says yes - all generics must meet strict standards for strength, purity, and how quickly they’re absorbed. But absorption can vary slightly, and for drugs with a narrow therapeutic index - like warfarin or levothyroxine - even small differences can matter. That’s why some doctors and patients prefer to stick with one version.
What should I do if I feel worse after switching to a generic?
Contact your pharmacist and doctor right away. Keep a log of your symptoms, when they started, and what medication you were taking before and after. Report the issue to the FDA’s MedWatch program. You have the right to ask for your original brand, and your doctor can write "dispense as written" on future prescriptions to prevent future switches.
Can I refuse a generic substitution even if my state requires it?
Yes. Even in states with mandatory substitution laws, your refusal overrides the law. You can say no at the pharmacy counter. You can also ask your doctor to write "dispense as written" on your prescription - this legally blocks substitution unless you change your mind later.
Why do some states ban substitution for certain drugs?
Thirteen states maintain lists of narrow therapeutic index (NTI) drugs that cannot be substituted without special permission. These include drugs like digoxin, phenytoin, and warfarin. These drugs have very little room for error - a small change in how your body absorbs them can lead to serious side effects. The bans are meant to protect patients, even if the FDA says the generics are equivalent.


12 Responses
So basically, we’re playing Russian roulette with our thyroid meds? 🤯 I take levothyroxine and I swear my mood swings are worse when they switch me to a new generic… like my brain’s on a rollercoaster made of confusion and caffeine. Why does the FDA say it’s ‘therapeutically equivalent’ but my body says ‘nope’? 🤔
Y’all, I get it - generics save money, and that’s amazing 💖 But if your life depends on that pill being *exactly* the same every time… why are we gambling with it? My mom had a seizure after a switch - not because the drug was bad, but because her body remembered the old one. Please, please, please ask your pharmacist. Don’t just assume. You’ve got rights. Say NO. Write it down. Protect yourself. 💪❤️
Pharmacists spend 12 minutes per script checking laws? That’s not efficiency. That’s chaos.
Hey, if you’re on warfarin or levothyroxine - don’t just nod and walk away. Ask for the brand name. Ask if it changed. Write it on your phone. Keep a little note in your wallet. I used to forget until I had a scary INR spike. Now I say: ‘Is this the same as last time?’ and I mean it. Your body notices differences before your brain does. Trust yourself. And if your doc writes ‘dispense as written’ - that’s your superpower. Use it. 🙌
I think the real issue isn’t the law - it’s the lack of communication. Pharmacists are overloaded. Patients are confused. Doctors are caught in the middle. We need better systems, not more rules. Maybe a simple QR code on the bottle that links to a page explaining the substitution? Or a standardized form? Something that doesn’t require memorizing 50 state laws just to get your meds.
Isn’t it ironic? We live in a world where algorithms predict our moods, yet we let a pharmacist in Nebraska decide whether my seizure meds are ‘equivalent’ to the one I’ve been on for 12 years? The body is not a spreadsheet. The soul remembers the pill. And when it doesn’t get the same one… it grieves. 🌑
generic = cheaper. brand = expensive. but sometimes… the difference is your life. i learned that the hard way. now i just ask. no big deal. pharmacy lady always looks at me like i’m weird. but i’m alive. so 🤷♀️
NTI drugs require precision pharmacokinetics. Generic bioequivalence thresholds (80–125%) are statistically acceptable but clinically negligent for narrow therapeutic index agents. The FDA’s equivalence paradigm is rooted in population-level metrics, not individual pharmacodynamic variance. This regulatory failure is systemic. You’re not paranoid - you’re pharmacologically literate.
Wait - so if I’m in a state that doesn’t require notification, and I get switched without knowing… and then I have a bad reaction… who’s liable? The pharmacist? The state? The FDA? This feels like a legal loophole dressed up as policy. I just want to know what’s in my hand before I swallow it. Is that too much to ask?
Let’s be real - 92.5% generic utilization doesn’t mean 92.5% patient satisfaction. The Orange Book is a regulatory fiction for complex molecules. Biosimilars aren’t generics. Interchangeability isn’t equivalence. And pharmacists aren’t clinicians. The system is built on assumptions, not evidence. And patients are the lab rats.
my grandma died because they switched her digoxin and no one told her. i don’t care if the science says it’s fine. my grandma didn’t die from bad science. she died from bad policy. and now i call every pharmacy before i pick up anything. even if it’s just ibuprofen. i’m not taking chances anymore. 🤕
Why are we letting 50 different states mess with life-saving meds? This isn’t freedom. It’s bureaucratic insanity. If you’re gonna make a national healthcare system, at least make the rules the same. Stop letting states play ‘Medication Roulette’ with people’s lives. We’re not a collection of 50 countries - we’re one nation. Fix this.